1 Avogadro
Answer 1: The Avogadro’s number full is equal to 6.02214076 × 10 23. Furthermore, Avogadro’s number refers to the number of particles that exist in one mole of any substance. Moreover, this number is also known as the Avogadro’s constant and is the number of atoms that are found to be existing in exactly 12 grams of carbon-12. This question requires an understanding of what avogadro's number actually represents. Avogadro's number, 6.022. 10 23 is the number of things in one mole. The question indicates that there is 1 mole of H 2. Thus there are 6.022. 10 23 molecules of H 2. However the question is asking for the amount of atoms in 1 mole of H 2.
1 Avogadro
What is the Avogadro Constant?
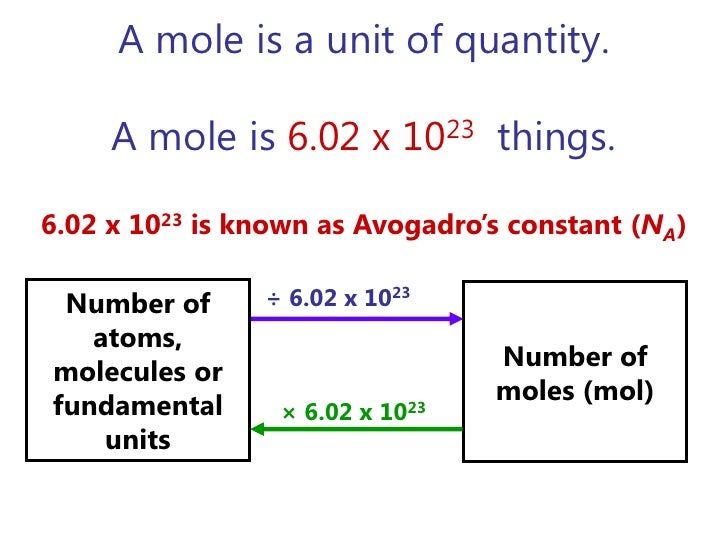
In Chemistry, the weight of a sample is directly proportional to the number of elementary entities in the sample. The Avogadro constant is used to express this relationship. The Avogadro constant or Avogadro’s number refers to the number of atoms, molecules, electrons, or ions contained in one mole of a substance. Its value is 6.0221415 × 1023 mol-1 (the number of atoms or molecules per mole).
1 Mole Avogadro

Amedeo Avogadro, who was an Italian scientist of the early nineteenth century, proposed this constant. The Avogadro constant is represented by the symbols N or NA and sometimes L (after the Austrian chemist Loschmidt, who first calculated the value of this constant). Before going into the details of this number and how it is derived, we need to know the definition of mole. One mole is defined as the amount of substance that contains as many atoms and molecules as there are entities in 12 grams of carbon-12. The entities can also be electrons, ions, and other subatomic particles. It is important to specify the entity that is being studied, for example if we are to determine the number of electrons in a substance, we would say a mole of electrons contained in that substance is equal to 6.02 × 1023 mol-1 electrons.
The Avogadro constant can be related to the atomic weight of a substance. Since, the atomic weight of carbon-12 is 12 we can say that the mass of one mole of carbon-12 is 12g. Similarly, as the molecular weight of H2O or water is 18, the mass of one mole of water is 18g. This provides another useful relation, which allows us to calculate the mass of one molecule of a substance. Hence, m = M/NA where M is the molecular mass of a substance, and NA is the Avogadro’s number.
1 Divided By Avogadro Number
Moreover, Avogadro’s number gives us relations with many other constants, the most significant of which is the definition of the unified atomic mass unit or Dalton (Da). The Dalton is a unit of mass, which expresses the approximate mass of a hydrogen atom, proton, or neutron. Hence, 1 u is equal to 1.660538782(83) × 10-24 g, which is obtained by dividing the molar mass constant (Mu) by the Avogadro constant NA. The value of the molar mass constant is 1×10–3 kg/mol in SI units.
The Avogadro constant is important because it provides scientists with a way to predict the amount and ratios of substances required to bring about a chemical reaction, a branch of chemistry known as Stoichiometry. Relating this huge number to the weight of a substance, we can estimate the number of molecules required to bring about a chemical change. Cisco anyconnect 4.8 mac download. It is sometimes known as the “chemist’s dozen” in layman's terms.
1 Avogadro Number
Avogadro is an advanced molecule editor and visualizer designed forcross-platform use in computational chemistry, molecular modeling,bioinformatics, materials science, and related areas. It offersflexible high quality rendering and a powerful pluginarchitecture.1 Avogadro Number
- Cross-Platform: Molecular builder/editor for Windows, Linux, and Mac OS X.
- Free, Open Source: Easy to install and all sourcecode and documentation is available to modify or extend.
- International: Translations into Chinese, French, German, Italian,Russian, Spanish, and others, with more languages to come.
- Intuitive: Built to work easily for students and advanced researchers both.
- Fast: Supports multi-threaded rendering and computation.
- Extensible: Plugin architecture for developers, including rendering, interactive tools, commands, and Python scripts.
- Flexible: Features include Open Babel import of chemical files, input generation for multiple computational chemistry packages, crystallography, and biomolecules.
- How to cite Avogadro: The Avogadro Paper
News
- Results from 2018 Community Survey
- 2018 Avogadro User Meeting
- Avogadro Part of Google Summer of Code 2018
- Support Avogadro through Open Collective
- Avogadro 1.90.0 Released
