Partial Charges
A partial charge is a non-integer charge value when measured in elementary charge units. Partial charge is more commonly called net atomic charge. It is represented by the Greek lowercase letter 𝛿, namely 𝛿− or 𝛿+.
A nonpolar molecule my have partial charges at different parts of the molecule, but the net dipole moment is zero. The dipole moment is the vector sum of the individual bond dipoles. Carbon dioxide is nonpolar but the oxygen atoms have a slight negative charge while the carbon has a slight positive charge. That is really a great question that should be asked much more frequently. There are multiple ways to calculate partial charges and all will give different values for reasons that will become obvious. A molecule is basically a collection of positi. Partial-month charges If you change your plan or add-ons in the middle of a bill period, you’ll see partial-month charges or credits on your next bill. Here’s how to calculate your partial-month charges For example, say you switch from a plan that costs $45 a month to one that costs $60.
Partial charges are created due to the asymmetric distribution of electrons in chemical bonds. For example, in a polar covalent bond like HCl, the shared electron oscillates between the bonded atoms. The resulting partial charges are a property only of zones within the distribution, and not the assemblage as a whole. For example, chemists often choose to look at a small space surrounding the nucleus of an atom: When an electrically neutral atom bonds chemically to another neutral atom that is more electronegative, its electrons are partially drawn away. This leaves the region about that atom's nucleus with a partial positive charge, and it creates a partial negative charge on the atom to which it is bonded.
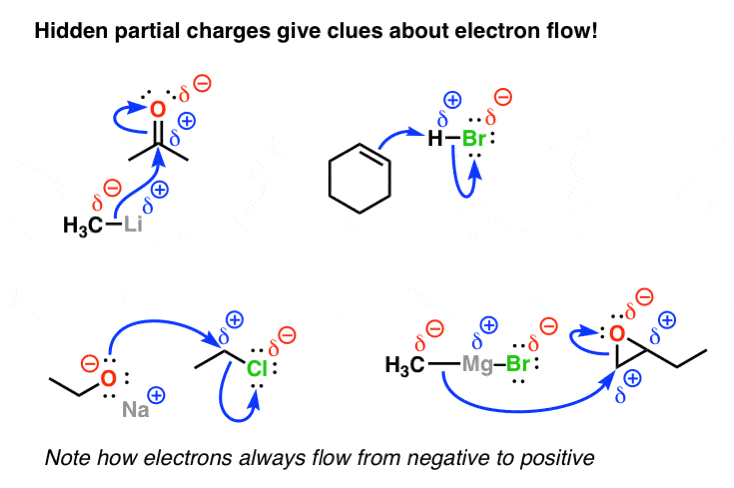
and of the related Grignard compound
with indication of the partial charge.
The bond has a partial positive charge, we make the “tail” of the arrow into a “plus” sign. Thus the very polar hydrogen fluoride bond (electronegativities: H = 2.1, F = 4.0) could be represented either by labeling the two atoms with the partial charge symbols or by drawing a dipole moment vector.
Partial Charge Example
In such a situation, the distributed charges taken as a group always carries a whole number of elementary charge units. Yet one can point to zones within the assemblage where less than a full charge resides, such as the area around an atom's nucleus. This is possible in part because particles are not like mathematical points—which must be either inside a zone or outside it—but are smeared out by the uncertainty principle of quantum mechanics. Because of this smearing effect, if one defines a sufficiently small zone, a fundamental particle may be both partly inside and partly outside it.
Uses[edit]
Partial Charges Definition
Partial atomic charges are used in molecular mechanicsforce fields to compute the electrostatic interaction energy using Coulomb's law, even though this leads to substantial failures for anisotropic charge distributions.[1] Partial charges are also often used for a qualitative understanding of the structure and reactivity of molecules.
Occasionally, δδ+ and is used to indicate a partial charge that is less positively charge than or δ+ (likewise for δδ-) in cases where it's relevant to do so.[2] This can be extended to δδδ+ to indicate even weaker partial charges as well. Generally, a single δ+ (or δ-) is sufficient for most discussions of partial charge in organic chemistry
Determining partial atomic charges[edit]
Partial atomic charges can be used to quantify the degree of ionic versus covalent bonding of any compound across the periodic table. The necessity for such quantities arises, for example, in molecular simulations to compute bulk and surface properties in agreement with experiment. Evidence for chemically different compounds shows that available experimental data and chemical understanding lead to justified atomic charges.[3] Atomic charges for a given compound can be derived in multiple ways, such as:
- extracted from electron densities measured using high resolution x-ray, gamma ray, or electron beam diffraction experiments
- measured dipole moments
- the Extended Born thermodynamic cycle, including an analysis of covalent and ionic bonding contributions
- spectroscopically measured properties, such as core-electron binding energy shifts
- the relationship of atomic charges to melting points, solubility, and cleavage energies for a set of similar compounds with similar degree of covalent bonding
- the relationship of atomic charges to chemical reactivity and reaction mechanisms for similar compounds reported in the literature.
The discussion of individual compounds in prior work has shown convergence in atomic charges, i.e., a high level of consistency between the assigned degree of polarity and the physical-chemical properties mentioned above. The resulting uncertainty in atomic charges is ±0.1e to ±0.2e for highly charged compounds, and often <0.1e for compounds with atomic charges below ±1.0e. Often, the application of one or two of the above concepts already leads to very good values, especially taking into account a growing library of experimental benchmark compounds and compounds with tested force fields.[4]
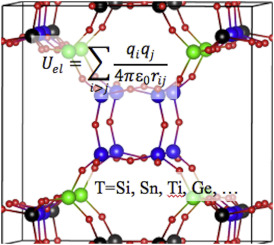
The published research literature on partial atomic charges varies in quality from extremely poor to extremely well-done. Although a large number of different methods for assigning partial atomic charges from quantum chemistry calculations have been proposed over many decades, the vast majority of proposed methods do not work well across a wide variety of material types.[5][6] Only as recently as 2016 was a method for theoretically computing partial atomic charges developed that performs consistently well across an extremely wide variety of material types.[5][6] All of the earlier methods had fundamental deficiencies that prevented them from assigning accurate partial atomic charges in many materials.[5][6] Mulliken and Löwdin partial charges are physically unreasonable, because they do not have a mathematical limit as the basis set is improved towards completeness.[7] Hirshfeld partial charges are usually too low in magnitude.[8] Some methods for assigning partial atomic charges do not converge to a unique solution.[5] In some materials, atoms in molecules analysis yields non-nuclear attractors describing electron density partitions that cannot be assigned to any atom in the material; in such cases, atoms in molecules analysis cannot assign partial atomic charges.[9]
According to Cramer (2002), partial charge methods can be divided into four classes:[10]
- Class I charges are those that are not determined from quantum mechanics, but from some intuitive or arbitrary approach. These approaches can be based on experimental data such as dipoles and electronegativities.
- Class II charges are derived from partitioning the molecular wave function using some arbitrary, orbital based scheme.
- Class III charges are based on a partitioning of a physical observable derived from the wave function, such as electron density.
- Class IV charges are derived from a semiempirical mapping of a precursor charge of type II or III to reproduce experimentally determined observables such as dipole moments.
The following is a detailed list of methods, partly based on Meister and Schwarz (1994).[11]
- Population analysis of wavefunctions
- Löwdin population analysis[12]
- Coulson's charges
- Natural charges[7]
- CM1, CM2, CM3, CM4, and CM5[13] charge models
- Partitioning of electron density distributions
- Bader charges (obtained from an atoms in molecules analysis)
- Density fitted atomic charges
- Hirshfeld charges[14]
- Maslen's corrected Bader charges[15]
- Politzer's charges
- Voronoi Deformation Density charges
- Density Derived Electrostatic and Chemical (DDEC) charges, which simultaneously reproduce the chemical states of atoms in a material and the electrostatic potential surrounding the material's electron density distribution[16][5]
- Charges derived from dipole-dependent properties
- Dipole charges
- Dipole derivative charges, also called atomic polar tensor (APT) derived charges,[17] or Born, Callen, or Szigeti effective charges [18]
- Charges derived from electrostatic potential
- Chelp
- Merz-Singh-Kollman (also known as Merz-Kollman, or MK)
- RESP (Restrained Electrostatic Potential)[19]
- Charges derived from spectroscopic data
- Charges from infrared intensities
- Charges from X-ray photoelectron spectroscopy (ESCA)
- Charges from X-ray emission spectroscopy
- Charges from X-ray absorption spectra
- Charges from ligand-field splittings
- Charges from UV-vis intensities of transition metal complexes
- Charges from other spectroscopies, such as NMR, EPR, EQR
- Charges from other experimental data
- Charges from bandgaps or dielectric constants
- Apparent charges from the piezoelectric effect
- Charges derived from adiabatic potential energy curves
- Electronegativity-based charges
- Other physicochemical data, such as equilibrium and reaction rate constants, thermochemistry, and liquid densities.
References[edit]
- Frank Jensen. Introduction to Computational Chemistry (2nd ed.). Wiley. ISBN978-0-470-01187-4.
- ^Kramer, Christian; Spinn, Alexander; Liedl, Klaus R. (2014). 'Charge Anisotropy: Where Atomic Multipoles Matter Most'. Journal of Chemical Theory and Computation. 10 (10): 4488–4496. doi:10.1021/ct5005565.
- ^'Basic principles in organic chemistry: Steric and electronic effects in a covalent bond – Open Teaching Project'. Retrieved 2020-10-11.
- ^H. Heinz; U. W. Suter (2004). 'Atomic Charges for Classical Simulations of Polar Systems'. J. Phys. Chem. B. 108: 18341–18352. doi:10.1021/jp048142t.
- ^H. Heinz; T. Z. Lin; R. K. Mishra; F. S. Emami (2013). 'Thermodynamically Consistent Force Fields for the Assembly of Inorganic, Organic, and Biological Nanostructures: The INTERFACE Force Field'. Langmuir. 29: 1754–1765. doi:10.1021/la3038846. PMID23276161.
- ^ abcdeT. A. Manz; N. Gabaldon-Limas (2016). 'Introducing DDEC6 atomic population analysis: part 1. Charge partitioning theory and methodology'(PDF). RSC Adv. 6: 47771–47801. doi:10.1039/c6ra04656h.
- ^ abcN. Gabaldon-Limas; T. A. Manz (2016). 'Introducing DDEC6 atomic population analysis: part 2. Computed results for a wide range of periodic and nonperiodic materials'. RSC Adv. 6: 45727–45747. doi:10.1039/c6ra05507a.
- ^ abA. E. Reed; R. B. Weinstock; F. Weinhold (1985). 'Natural population analysis'. J. Chem. Phys. 83: 735–746. Bibcode:1985JChPh..83..735R. doi:10.1063/1.449486.
- ^E. R. Davidson; S. Chakravorty (1992). 'A test of the Hirshfeld definition of atomic charges and moments'. Theor. Chim. Acta. 83: 319–330. doi:10.1007/BF01113058.
- ^C. Gatti; P. Fantucci; G. Pacchioni (1987). 'Charge density topological study of bonding in lithium clusters'. Theor. Chim. Acta. 72: 433–458. doi:10.1007/BF01192234.
- ^C. J. Cramer (2002). Essentials of Computational Chemistry: Theories and Methods. Wiley. pp. 278–289.
- ^J. Meister; W. H. E. Schwarz (1994). 'Principal Components of Ionicity'. J. Phys. Chem. 98: 8245–8252. doi:10.1021/j100084a048.
- ^'Q-Chem 4.3 User's Manual : Wavefunction Analysis'. Retrieved 2017-07-23.CS1 maint: discouraged parameter (link)
- ^A. V. Marenich; S. V. Jerome; C. J. Cramer; D. G. Truhlar (2012). 'Charge Model 5: An Extension of Hirshfeld Population Analysis for the Accurate Description of Molecular Interactions in Gaseous and Condensed Phases'. J. Chem. Theory Comput. 8: 527–541. doi:10.1021/ct200866d.
- ^F. L. Hirshfeld (1977). 'Bonded-atom fragments for describing molecular charge densities'. Theor. Chim. Acta. 44: 129–138. doi:10.1007/BF00549096.
- ^E. N. Maslen; M. A. Spackman (1985). 'Atomic charges and electron density partitioning'. Aust. J. Phys. 38: 273–287. Bibcode:1985AuJPh..38..273M. doi:10.1071/PH850273.
- ^T. A. Manz; D. S. Sholl (2012). 'Improved Atoms-in-Molecule Charge Partitioning Functional for Simultaneously Reproducing the Electrostatic Potential and Chemical States in Periodic and Nonperiodic Materials'. J. Chem. Theory Comput. 8 (8): 2844–2867. doi:10.1021/ct3002199.
- ^P. J. Stephens; K. J. Jalkanen; R. W. Kawiecki (1990). 'Theory of vibrational rotational strengths: comparison of a priori theory and approximate models'. J. Am. Chem. Soc. 112 (18): 6518–6529. doi:10.1021/ja00174a011.
- ^Ph. Ghosez; J.-P. Michenaud; X. Gonze (1998). 'Dynamical atomic charges: The case of ABO3 compounds'. Phys. Rev. B. 58 (10): 6224–6240. arXiv:cond-mat/9805013. Bibcode:1998PhRvB..58.6224G. doi:10.1103/PhysRevB.58.6224.
- ^C. I. Bayly; P. Cieplak; W. Cornell; P. A. Kollman (1993). 'A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model'. J. Phys. Chem. 97 (40): 10269–10280. doi:10.1021/j100142a004.
Partial Charges Are Present In Which Type Of Bond
